Research
Sustainable Catalysis & Asymmetric Synthesis
The goal of our research program is to discover and study new metal-catalyzed reactions with the aim to develop highly selective, sustainable and atom-efficient synthetic methodologies based on the use of readily accessible materials and the minimization of waste production. In this context, the selective functionalization of hydrocarbons and the development of catalytic enantioselective reactions and its application in the synthesis of bioactive compounds play an important role.
We are currently active in following research lines:
Alkane functionalization (ERC CoG “BECAME”)
 Alkanes are very abundant in our planet. This natural abundance makes its use as chemical feedstocks very attractive. However, their inertness in terms of bond dissociation energy, ionization potential and pKa has rendered the use of methane extremely difficult for purposes beyond aerobic combustion and the production of syngas. Thus, the activation and functionalization of the simplest alkane represents a major challenge.
Alkanes are very abundant in our planet. This natural abundance makes its use as chemical feedstocks very attractive. However, their inertness in terms of bond dissociation energy, ionization potential and pKa has rendered the use of methane extremely difficult for purposes beyond aerobic combustion and the production of syngas. Thus, the activation and functionalization of the simplest alkane represents a major challenge.
The direct use of alkanes in organic transformations could lead to clean and highly atom-efficient processes as the formation of inorganic waste, usually generated when organometallic reagents are used, would be eliminated in these cases. We work on development of new technologies based on metal catalysts which enable the conversion of simple alkanes such as e.g. methane into more complex organic molecules will lead to cleaner and more sustainable synthetic avenues for the production of highly valuable chemicals.
 The project “BECAME – Bimetallic Catalysis for Diverse Methane Functionalization” has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement No. 863914)
The project “BECAME – Bimetallic Catalysis for Diverse Methane Functionalization” has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement No. 863914)
Catalytic Carboboration of Unsaturated Hydrocarbons
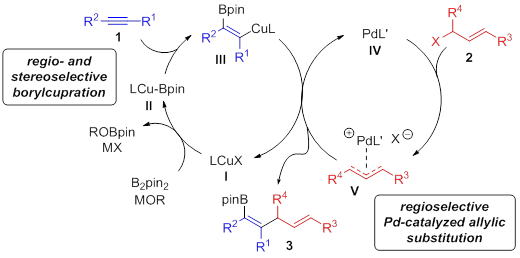
Traditional strategies for the cross coupling of Csp2-nucleophiles typically involve the pre-generation, and often the isolation, of a stoichiometric amount of the organometallic reagent. To circumvent this problem, we envisioned a different approach that involves the use of alkynes as pro-nucleophiles by catalytic generation of Csp2-organometallic species via addition of a LnCu-Bpin complex across the unsaturated hydrocarbon followed by cross-coupling. By using this strategy under single copper or bimetallic catalysis regime, we have reported highly chemo-, regio- and enantioselective transformations in which both positions of the alkyne are functionalized while an additional site (Bpin) for further functionalization is incorporated.
Approach 1: Cu catalysis
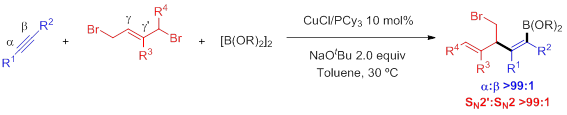
Angew. Chem. Int. Ed. 2018, 57, 9945
Angew. Chem. Int. Ed. 2019, 58, 18230
Approach 2: Synergistic Cu/Pd catalysis